Artificial Intelligence and Alzheimer’s: Transforming Patient Screening with Predictive Models
Exploring the impact of a new predictive artificial intelligence model to better predict clinical outcomes and improve Alzheimer’s disease diagnostics for better clinical trial recruitment and retention.
Alzheimer’s disease (AD) remains one of the most challenging medical conditions to diagnose and treat effectively. Currently, there is no universally accessible screening technique for diagnosing AD, largely due to the complex nature of making a diagnosis involving costly, invasive, procedures within specialized clinical environments. As a result, AD clinical trials are notably slow in recruiting participants, are more time-consuming to carry out, and are costlier compared to those in many other therapeutic areas. In addition, around 99% of patients eligible for an AD clinical trial never get referred to participate in trials.
Despite these challenges, major pharmaceutical companies continue to invest heavily in AD research, reflecting the ongoing need for better treatment options. In the past few years, two drugs branded as ‘disease modifiers’ (Aduhelm™ and Leqembi™) were approved by the FDA, although none outside of the US. However, both encountered significant challenges post-approval, including safety concerns, complex administration requirements, and costly safety monitoring processes. Aduhelm™ was withdrawn from the market, while Leqembi™ has not yet achieved commercial success. The recent setbacks in AD drug development highlight the critical need for innovations in trial design and patient recruitment.
Addressing Screen Failure Rate Challenges in AD Clinical Trials
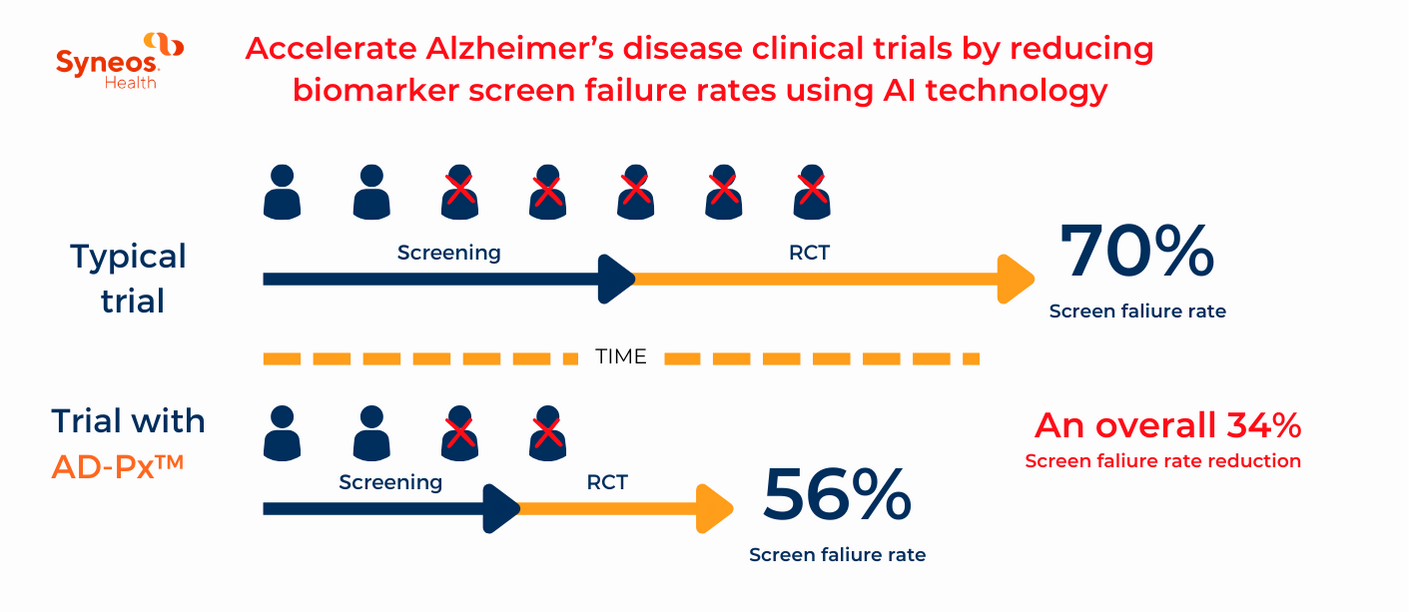
A primary challenge in early AD clinical trial recruitment is the high screen failure rate, often around 70%, which dramatically slows down the screening process. FDA revised draft guidelines on early AD emphasizes the need for clinical efficacy and the use of surrogate biomarkers, which further complicate patient screening and recruitment.
Enter AD-Px™—a new artificial intelligence (AI) tool shown to reduce screen failure rates by accurately predicting which patients are likely to show cognitive decline within two years. Predictive models mean researchers do not merely sift through patient data but instead can revolutionize patient eligibility, ensuring a more targeted and efficient recruitment process.
Predictive models mean researchers do not merely sift through patient data but instead can revolutionize patient eligibility, ensuring a more targeted and efficient recruitment process.
Earlier AI applications in AD research, typically focused on early detection through changes in voice, retinal scans, or imaging analysis, were not universally applicable across AD clinical trials. AI can now integrate multiple aspects of AD diagnostics and patient assessment into a single predictive model, designed to work seamlessly with existing Alzheimer’s trial recruitment assessments.
Creating a Cohesive Risk Profile to Improve Trial Recruitment
By incorporating widely used cognitive tests such as the Mini Mental State Exam (MMSE) and the Clinical Dementia Rating (CDR) Sum of Boxes, along with genetic risk factors, biomarkers, amyloid burden confirmation and demographic data predictive models, researchers can streamline the screening process and ensure that only patients most likely to benefit are enrolled in trials, thereby reducing costs and enhancing the potential for meaningful outcomes. By making integrated screening methods universally applicable without increasing the burden on patients or costs for sites, the new screening methods can be easily integrated into current clinical practices without disruption, further simplifying the transition towards more advanced, AI-driven methodologies.
The use of AI to synthesize and analyze these diverse data points into a cohesive risk profile represents a significant leap forward. It allows for a more precise selection of trial participants, without lowering cut-off for biomarkers, reducing the need to screen large numbers of potential participants and thereby addressing one of the most time-consuming and costly aspects of early AD clinical trials. By enabling faster enrollment and minimizing costs, this technology paves the way for more rapid clinical development and potentially quicker delivery of new treatments to patients.
Pushing the Boundaries of AD Research
As AI allows us to push the boundaries of what is possible in Alzheimer’s disease research, it is possible to not only enhance one’s competitive edge but also contribute significantly to the global quest for effective Alzheimer’s treatments. The innovation brought on by a partnership between technology and neuroscience disease specialists is setting new standards in the AD clinical trial domain, demonstrating how integrating AI can address complex challenges and lead to more efficient and effective trials. This approach not only aligns with but actively advances FDA guidelines, paving the way for quicker, more reliable paths to treatment discovery and ultimately, offering hope for better management of Alzheimer’s disease.
Learn more about how the Neuroscience team at Syneos Health can help you apply customized therapeutic strategies, processes and technologies to facilitate more efficient trials, designed to improve the likelihood of regulatory and commercial success.
Contributors
Claudine Brisard, MD, Senior Vice President of Medical and Scientific Management
Roberta Anderson, TSI, Senior Vice President of Clinical Development (CNS)
Christine Maurette, DPD, Executive Director of Project Delivery




