Premières phases
Aussi dynamique qu’exigeant
Les données humaines initiales sont une étape importante dans le processus de développement de médicaments.
Depuis plus de 25 ans, notre équipe spécialisée dans les Premières phases se consacre à l’élaboration de solutions sur mesure pour cette étape exigeante.

Solutions sur mesure, du début à la fin
Qu’il s’agisse de premiers essais sur les humains, de l’interaction médicamenteuse, de l’insuffisance rénale ou hépatique ou d’études de validation de concept, nous sommes en mesure de mener toutes les études précliniques, de la phase I à la phase IIa, notamment : premiers essais sur les humains (FIH), preuve de concept, biodisponibilité/bioéquivalence (BA/BE), interaction médicamenteuse (DDI), déficience organique, études approfondies (TQT), biosimilaire, et divers essais pharmacocinétiques (PK), dose unique croissante/dose multiple croissante (SAD/MAD).
Nous disposons également d’une solide expérience dans l’utilisation d’un large éventail de produits de recherche (PR), y compris ceux d’origine biologique, et de multiples méthodes d’administration. En outre, nous avons accès à des populations de patients spécifiques, dont les Japonais, les Chinois et les patients obèses ou âgés.
Notre expertise comprend :

Aucun formulaire 483 émis dans nos cliniques
Réseau de collaboration entre les centres externes de l’Asie-Pacifique/l’Union européenne/l’Amérique du Nord
Nous travaillons en prolongement de votre équipe et fournissons des options tactiques adaptées au développement précoce de votre produit et vous guidons rapidement vers une démonstration de faisabilité et des résultats substantiels.
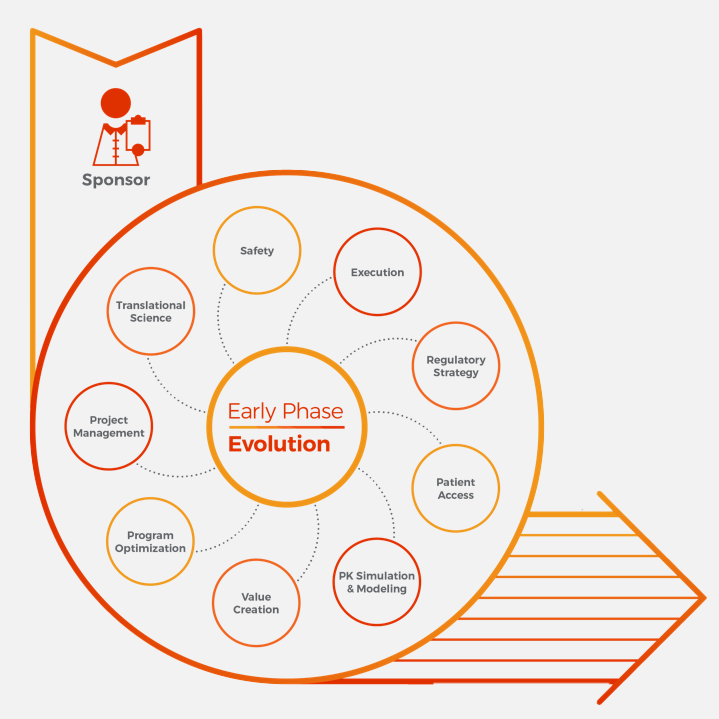
Une expertise spécialisée pour relever tous les défis
- Pour faciliter la prise de décision sur les prochaines étapes de votre programme de développement de médicaments, notre groupe de pharmacométrie fournit des capacités d’extraction de données pour tous vos besoins de modélisation et d’analyse quantitatives.
- Notre équipe spécialisée dans la biométrie offre une gamme complète de services, dont la pharmacologie clinique, la gestion des données, les statistiques, la rédaction médicale et le service de conseil en réglementation.
- Notre équipe de Solutions bioanalytiques propose plus de 1 200 tests prêts à être utilisés, ce qui fait de Syneos Health® un des trois premiers fournisseurs mondiaux de solutions bioanalytiques.

Exécution impeccable et planification de la suite des événements
Nous adoptons une approche évoluée et multidisciplinaire du développement clinique des premières phases. Nous nous concentrons rigoureusement sur l’excellence opérationnelle, mais nous sommes en mesure d’engager des experts en stratégie de développement de produits, en création de valeur, en accès aux sujets/patients et dans d’autres disciplines pour soutenir votre planification de la phase initiale.

Modèle unique de collaboration de Syneos Health
Notre approche stratégique de la recherche de phase I consiste à obtenir des informations sur les patients plus tôt dans le processus de développement clinique. Nous avons développé trois modèles de collaboration uniques qui favorisent le partenariat avec des chercheurs sélectionnés et leurs patients afin de motiver la participation et l’engagement, tout en intégrant une équipe et des installations expérimentées pour assurer une supervision et une exécution efficaces de l’étude.
Modèle 1 : Partenariat et tâches partagées
- Certains éléments sont menés au centre de recherche secondaire, comme le recrutement et la sélection, alors que d’autres sont menés dans notre unité de service de phase I par notre personnel avec le chercheur secondaire présent.
Modèle 2 : Diviser pour régner
- Une partie de l’étude est réalisée au centre de notre collaborateur, et le reste à notre unité de services de phase I, en plus de tous les services des organismes de recherche sous contrat
Modèle 3 : Réseau d’études en clinique externe
- Livraison de la faisabilité du centre, de la sélection et de la gestion, et soutien des chercheurs pour la conduite de l’étude, ainsi que tous les services périphériques d’organismes de recherche sous contrat.

Des renseignements sur le premier patient, plus rapidement
Notre approche stratégique de la recherche de Phase I consiste à obtenir des informations sur les patients plus tôt dans le processus de développement clinique. Notre expérience fournit les services et les solutions dont vous avez besoin pour que votre essai réussisse, peu importe le domaine de traitement. En savoir plus sur notre expertise en oncologie de Premières phases et la manière dont elle soutient notre engagement à fournir de nouveaux traitements contre le cancer aux patients.

Une portée mondiale, adaptée à vos besoins
Quelle que soit la taille de votre organisation et où que vous soyez dans le monde, notre portée mondiale combinée à notre approche collaborative du partenariat vous offre un soutien complet sous forme de service personnalisé.
- Nous sommes présents partout dans le monde avec des bureaux à Miami (États-Unis), à Québec (Canada), à Princeton (États-Unis), à Sophia-Antipolis (FRA) et, grâce à un réseau d’unités externes, en Europe et en Asie-Pacifique.
- Nos installations combinées donnent accès à trois environnements soumis à des réglementations différentes dans quatre zones géographiques distinctes : Asie-Pacifique, Europe, Canada et États-Unis.




