Healthcare Equity
What can individual brands and companies do to ensure equal access to scientific discovery and long-trusted therapies?
2021 Health Trends

In healthcare so much can seem unequal—from access to care to participation in clinical trials, but we’ve reached a critical inflection point for change. Today, we are seeing our industry act on behalf of equality. Leading life science organizations have teams dedicated to inclusion and diversity.
Clinical organizations are actively working to build both trust and awareness while making trials more portable and flexible to enable people to participate from anywhere.
For the first time, our industry is truthfully acknowledging our own harsh realities, signaling an opportunity to do better. Clinical trials are part of a continuum, and when our healthcare system fails minority populations by every metric, trial representation reflects the dereliction. We must embrace an ideal that is simple in essence: Study new medications in patients who represent the burden of disease. It is not about box-checking quotas. We must be intentional in our trial designs—listening, acknowledging, and maintaining momentum even after the pandemic subsides.
One promising sign: our commercial organizations are now focusing on how social determinants affect outcomes and creating programs that build the right environments for success.
Download the full Healthcare Equity trend report to learn what companies can do to increase diversity in clinical research, ensure equal access to scientific discovery, and together forge an inclusive healthcare ecosystem.
Did you know...
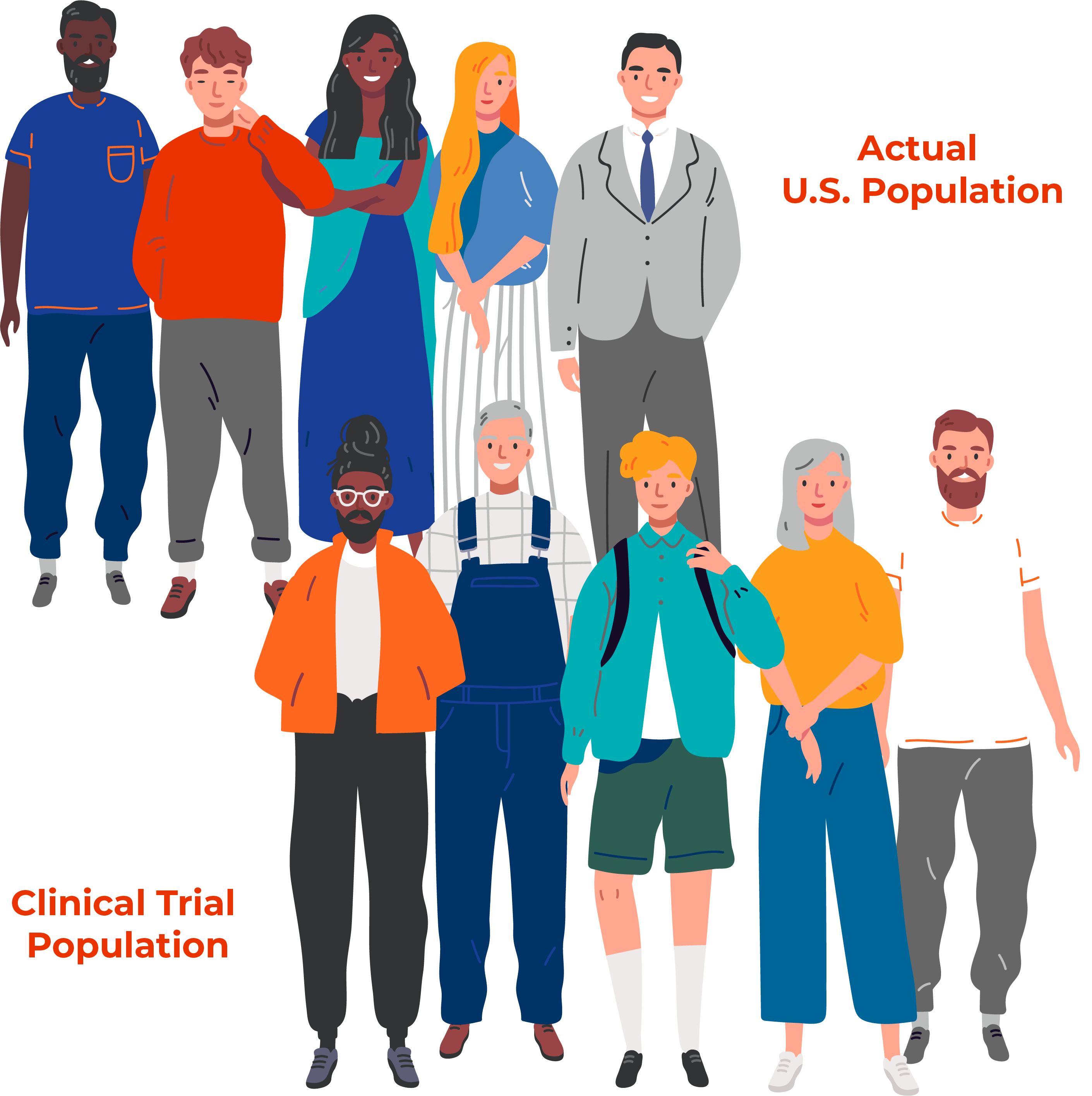
78%: The vast majority of clinical trial participants in the United States are Caucasian, despite the fact minorities make up 40% of the population. On average, 78% of participants in trials for new drugs were White and only 16% were Black or African American.
— FDA, 2020
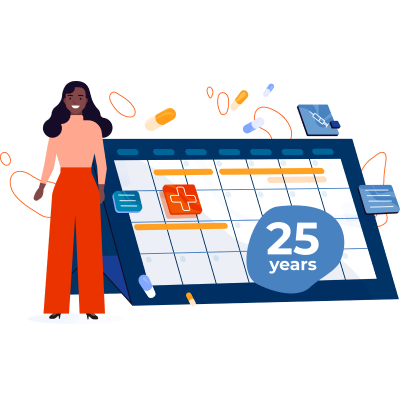
25 Years: It’s been a quarter of a century since the NIH mandated the inclusion of women and minority groups in all NIH-funded clinical research in a manner that is appropriate to the scientific question under study.
— NIH, 2020





