A Holistic Approach to Genetic Medicines is Next for the Life Science Industry
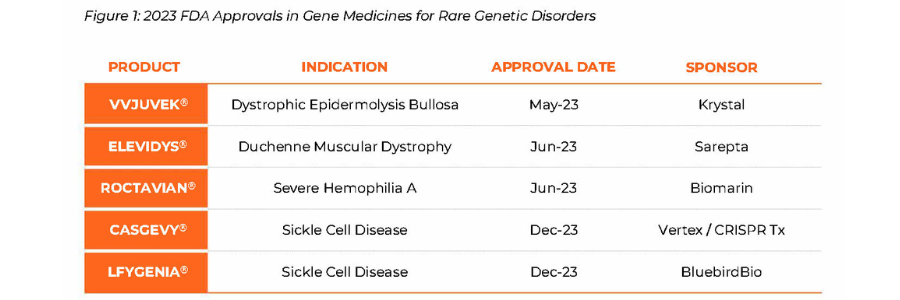
The case for pioneering novel applications of cell and gene therapies in 2024 could not be stronger. Fueled by FDA’s approval of five breakthrough gene therapies in 2023, the genetic medicine landscape in 2024 remains promising with a combination of scientific and regulatory readouts that are poised to combat a wide range of rare diseases (Figure 1). Health Canada’s approval of Pfizer’s Beqviz for the treatment of Hemophilia B in the first week of 2024 is an example of new therapies being approved globally.
The life science industry must adopt a holistic, fully integrated approach to balance the excitement of innovation against the operational realities in bringing complex therapies to patients with rare diseases or often smaller genetic subpopulations of larger diseases. Here are three critical areas underlying the efficient design and execution of sustainable genetic medicines.
Clinical
- Early clinical trials can combine different phases and can be set up to identify optimal safety and dosing escalation followed by dose expansion. This would allow the evolving paradigm of genetic medicine trials to result in smaller and faster trails that precisely target patients with a genetic component of a disease.
- The selection of key endpoints in a genetic medicine trial should be driven by patient, regulatory and payer considerations to ensure the greatest chance of both scientific and commercial success. In the case for rare genetic diseases, a critical enabler is a high-level of interaction with patient communities to build medicines that impact the disease and quality of life.
Operational
- A complete set of patient services designed bespoke for each disease is a critical enabler of accelerating patient recruitment and increasing the likelihood of meeting clinical trial timelines and milestones. While it's true that many of the regulatory setup requirements of a trial have been well discussed and that most genetic medicine clinical trials involve fewer patients, a robust suite of patient services can greatly remove barriers associated with the often-overlooked aspects of the patient and academic site experience. This includes indication specific patient services specifically designed for genetic medicine trial during the pre-treatment, treatment, short-term post-treatment, and long-term follow-up paradigm.
Commercial
- Building patient and commercial thinking early into clinical trial design ensures that the value of breakthrough medicines is recognized and adopted by the patient and payer community. To identify where a precise supply chain is needed for potentially one-time and high-priced genetic medicines, we recommend a broad commercial supply chain, balanced with comprehensive genetic medicines and logistical experience to inform commercial planning.
- Delivering breakthrough genetic medicines requires a unique commercial model informed by an exhaustive set of analytics to ensure patient and shareholder success. Sponsors have the additional challenge to educate the healthcare ecosystem on the paying for potentially one-time treatments while monitoring long-term patient outcomes. A nimble but targeted genetic medicine commercial model that accounts for complex logistics to qualified treatment centers that have the administration capabilities to treat patients is a must-have for any biotech looking to maximize extraordinary therapeutic outcomes.
The convergence of scientific advancements and regulatory milestones is propelling the biopharma industry with unprecedented possibilities. Striking a balance between scientific zeal and operational pragmaticism is paramount, particularly when navigating the complexities of bringing life-saving therapies to patients with rare diseases or smaller genetic subpopulations. 2024 demands a holistic yet balanced approach that considers multiple aspects of clinical, operational and commercial capabilities to ensure breakthroughs in genetic medicines are achieved.
Are your experts looking for ways to streamline and optimize their genetic medicine projects? Reach out to our team to explore more opportunities.
Contributors
Abhi Gupta | Senior Vice President, Head of Cell & Gene Therapy
Patrick Melvin | Vice President, Therapeutic Strategy Innovation




